ONCOLOGY Diagnostics
ONCOLOGY Diagnostics
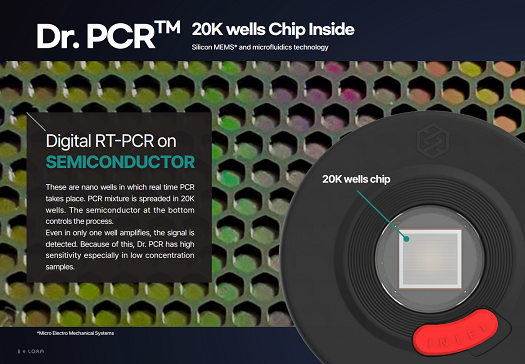
Cancer can hit anyone at any time and treatment remains a real challenge. Because cancer doesn’t follow rules. It fights back against therapies. It adapts. It changes its path. It does whatever it can to stay ahead of us. At the advanced edge of oncology, rapid access to accurate data about relevant cancer mutations and treatment resistance is vital and creates the opportunity for early disease interception reducing the anxiety while waiting for results and the time before starting the best possible treatment. Current technologies in molecular oncology are complex, require a lot of hands-on time and are often difficult to implement in the local laboratory. As a consequence, most laboratories do not perform molecular tests in-house, but send them out to specialized centers, where samples are batched in order to optimize costs. This causes delay to the fast delivery of results, preventing rapid initiation of correct therapy. In the meantime the tumor grows, which is detrimental in case of aggressively growing cancers. Fast initiation of immunotherapy or targeted therapy as first-line treatment is crucial for cancer patients, as it increases overall survival rates. Timely detection of biomarkers therefore is very important. Today, turnaround times of reference technologies are on average 18 days, with 14% of patients waiting longer than a month to be able to start treatment. Ninety-five percent of the patients have to wait more than a week in order to receive the biomarker results. This means that precious time is lost whereas treatment initiation could have been started and unnecessary use of chemotherapy with its side effects could have been avoided. Idylla, a fully automated, sample-to-result PCR based molecular diagnostics system, provides same-day results enabling physicians to make timely decisions on patients’ therapy. Idylla can be used with multiple sample types, including solid and liquid biopsies. This flexibility allows use of the system for diagnostic, research, and potentially future monitoring applications. Idylla, with its compact scalable design and outstanding ease-of-use, overcomesthe traditional barriers of molecular diagnostics, allowing it to be used in virtually any laboratory setting CML is a malignancy of the myeloid cell lineage characterized by a recurrent chromosomal abnormality: the Philadelphia chromosome, which results from the reciprocal translocation of the chromosomes 9 and 22. The Philadelphia chromosome contains a fusion gene called BCR-ABL1. The BCR-ABL1 codes for an aberrantly functioning tyrosine kinase that drives the malignant proliferation of the founding clone. The Dr. PCR™ BCR-ABL1 Major IS Detection Kit is a molecular in vitro diagnostic test that detects BCR-ABL1 fusion gene in leukemias including CML. The kit uses nucleic acid amplification based on a dual labeled hydrolysis probe, TaqMan and target specific primers. "Digital RT-PCR on SEMICONDUCTOR 20K wells chip Silicon MEMS* and microfluidics technology. These are nano wells in which real time PCR takes place. PCR mixture is spreaded in 20K wells. The semiconductor at the bottom controls the process. Even in only one well amplifies, the signal is detected. Because of this, Dr. PCR has high sensitivity especially in low concentration samples."THE NEED FOR IMPROVED, STANDARDIZED AND FAST DIAGNOSTICS

THE NEED FOR A RAPID TREATMENT INITIATION RESPONSE TOWARDS PATIENTS

IDYLLA, THE NEXT LEVEL IN DISEASE INTERCEPTION
Fast diagnostics, allow faster initiation of targeted therapy for cancer patients. Idylla, Biocartis’ fully automated molecular diagnostics system, is the first and only molecular diagnostic system that combines unsurpassed ease-of-use, speed and accuracy on multiple sample types. With its compact, scalable design and outstanding ease-of-use, Idylla overcomes the traditional barriers of molecular diagnostics, allowing it to be used in virtually any laboratory setting. And by providing same-day-results, Idylla enables physicians to make timely decisions on patients’ therapy.

LOAA ANALYZER - DIGITAL PCR
24 January 2025
Viewed 8250 times